Here are the essential concepts you must grasp in order to answer the question correctly.
Ethanol Dehydration
Heating an alcohol with sulfuric acid typically leads to dehydration, where water is removed, resulting in the formation of alkenes. In the case of symmetrical ethers, the reaction can favor the formation of a single product due to the equal reactivity of the alcohols involved. However, this process is less effective for unsymmetrical ethers, as the presence of different alcohols can lead to multiple products and a mixture of ethers.
Recommended video:
General Reaction of Dehydration with POCl3
Reactivity of Alcohols
Different alcohols have varying reactivities based on their structure (primary, secondary, or tertiary). When preparing unsymmetrical ethers, the reactivity of the alcohols can lead to preferential formation of one ether over another, complicating the reaction. This variability makes it difficult to control the outcome, resulting in a mixture of products rather than a single desired unsymmetrical ether.
Recommended video:
Ether Formation Mechanism
The mechanism for ether formation typically involves the nucleophilic attack of an alcohol on a carbocation intermediate. In the case of symmetrical ethers, the reaction can proceed smoothly due to the uniformity of the reactants. However, for unsymmetrical ethers, the formation of different carbocations from the two distinct alcohols can lead to competing pathways, making it challenging to achieve a clean synthesis of the desired ether.
Recommended video:
The Mechanism of Williamson Ether Synthesis.
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution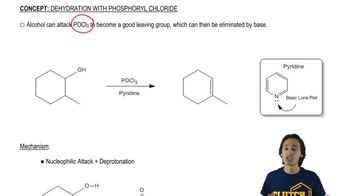



 4:33m
4:33m