Here are the essential concepts you must grasp in order to answer the question correctly.
Sonogashira Reaction
The Sonogashira reaction is a cross-coupling reaction that involves the coupling of an alkyne with an aryl halide using a palladium catalyst and a base, typically triethylamine. This reaction is significant in organic synthesis for forming carbon-carbon bonds, particularly in the construction of complex molecules and materials.
Recommended video:
Sonogashira Coupling Reaction
Alkynes
Alkynes are hydrocarbons that contain at least one carbon-carbon triple bond. They are characterized by their linear geometry and are generally more reactive than alkenes and alkanes due to the presence of the triple bond, which can participate in various chemical reactions, including the Sonogashira reaction.
Recommended video:
Aryl Halides
Aryl halides are organic compounds that consist of an aromatic ring bonded to a halogen atom (such as chlorine, bromine, or iodine). In the context of the Sonogashira reaction, aryl halides serve as one of the coupling partners, providing a reactive site for the formation of new carbon-carbon bonds with alkynes.
Recommended video:
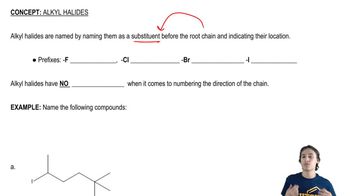
How to name alkyl halides
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution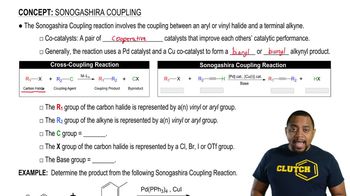


 2:54m
2:54m