Here are the essential concepts you must grasp in order to answer the question correctly.
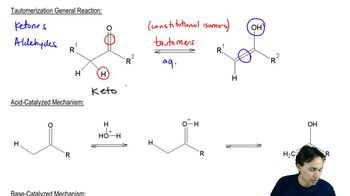
Vinyl Alcohols
Vinyl alcohols are a class of organic compounds characterized by the presence of a hydroxyl group (-OH) attached to a carbon-carbon double bond (C=C). They are typically unstable due to their tendency to tautomerize, meaning they can rapidly convert to more stable forms, such as carbonyl compounds (aldehydes or ketones). This instability is a key factor in understanding their reactivity and the mechanisms involved in their isomerization.
Recommended video:
Vinyl alcohols yield tautomers.
Tautomerization
Tautomerization is a chemical reaction that involves the rearrangement of atoms in a compound, resulting in the interconversion between two isomers, typically involving a shift of a hydrogen atom and a change in the position of a double bond. In the case of vinyl alcohols, tautomerization leads to the formation of carbonyl compounds, which are generally more stable. Understanding this process is crucial for proposing mechanisms for the isomerization of vinyl alcohols.
Recommended video:
Tautomerization Mechanisms
Mechanisms of Isomerization
The mechanisms of isomerization refer to the step-by-step pathways through which a compound transforms into its isomer. For vinyl alcohols, this often involves proton transfer, bond breaking, and bond formation, which can occur through various mechanisms such as acid-catalyzed or base-catalyzed pathways. Analyzing these mechanisms helps in predicting the products and understanding the conditions under which isomerization occurs.
Recommended video:
Monosaccharides - D and L Isomerism
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution