Here are the essential concepts you must grasp in order to answer the question correctly.
Systematic Nomenclature
Systematic nomenclature in organic chemistry refers to the standardized method of naming chemical compounds based on their structure and functional groups. The International Union of Pure and Applied Chemistry (IUPAC) provides rules for naming compounds, which include identifying the longest carbon chain, numbering the carbon atoms, and indicating the presence of functional groups. This systematic approach ensures that each compound has a unique and universally accepted name.
Recommended video:
Nomenclature of Heterocycles Concept 2
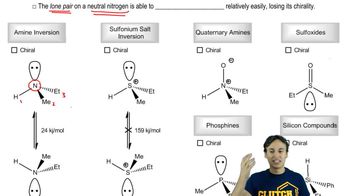
Chirality and Asymmetric Centers
Chirality is a property of a molecule that has non-superimposable mirror images, often due to the presence of asymmetric centers, typically carbon atoms bonded to four different substituents. These centers can exist in two configurations, designated as 'R' (rectus) or 'S' (sinister), based on the Cahn-Ingold-Prelog priority rules. Understanding chirality is crucial for determining the specific three-dimensional arrangement of atoms in a molecule, which can significantly affect its chemical behavior and biological activity.
Recommended video:
Understanding Other Chiral Atoms
D- and L- Configuration
The D- and L- configuration is a system used to classify sugars and amino acids based on their stereochemistry. For carbohydrates, the 'D' designation indicates that the hydroxyl group on the highest-numbered chiral carbon is on the right in a Fischer projection, while 'L' indicates it is on the left. This classification is essential for understanding the structure and reactivity of sugars like d-glucose, as it relates to their biological roles and interactions.
Recommended video:
Representations of L-Configuration
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 3:11m
3:11m