Here are the essential concepts you must grasp in order to answer the question correctly.
Lone Pairs
Lone pairs are pairs of valence electrons that are not involved in bonding and are localized on a single atom. They play a crucial role in determining the geometry and reactivity of molecules. Understanding how to identify and represent lone pairs is essential for accurately drawing Lewis structures and predicting molecular shapes.
Recommended video:
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They provide a visual way to understand the arrangement of electrons and the connectivity of atoms. Mastery of drawing Lewis structures is fundamental in organic chemistry for predicting molecular behavior and reactivity.
Recommended video:
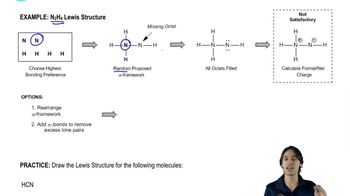
Drawing the Lewis Structure for N2H4.
Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom that are involved in forming bonds with other atoms. The number of valence electrons determines how an atom can bond and interact with others. Knowing how to count and distribute valence electrons is critical for constructing accurate molecular representations and understanding chemical reactivity.
Recommended video:
Valence Electrons of Transition Metals
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:14m
2:14m