Here are the essential concepts you must grasp in order to answer the question correctly.
Amine Synthesis
Amine synthesis involves various chemical reactions to produce amines, which are organic compounds containing nitrogen. Common methods include reductive amination, where a carbonyl compound reacts with an amine in the presence of a reducing agent, and nucleophilic substitution, where a leaving group is replaced by an amine. Understanding the structure of the target amine and the reactivity of the starting materials is crucial for selecting the appropriate synthesis route.
Recommended video:
Functional Group Transformation
Functional group transformation refers to the process of converting one functional group into another through chemical reactions. In the context of amine synthesis, this may involve converting a carbonyl group (like an aldehyde or ketone) into an amine. Recognizing how different functional groups can be interconverted is essential for designing a synthetic pathway that leads to the desired amine product.
Recommended video:
Identifying Functional Groups
Reagents and Conditions
The choice of reagents and reaction conditions is critical in organic synthesis, as they influence the reaction's efficiency and selectivity. For amine synthesis, common reagents include reducing agents like sodium borohydride or lithium aluminum hydride, and conditions such as temperature and solvent can significantly affect the reaction outcome. A thorough understanding of these factors helps in optimizing the synthesis process to achieve the desired amine.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution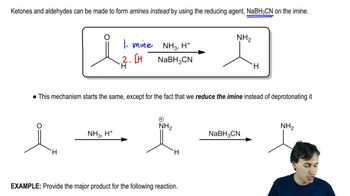



 3:46m
3:46m