Textbook Question
There are eight different five-carbon alkyl groups.
a. Draw them. b. give them systematic names.
628
views
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution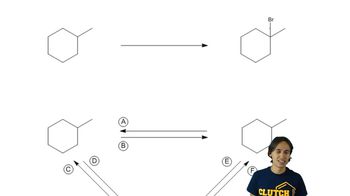

 6:42m
6:42mMaster Understanding Non-IUPAC Substituents with a bite sized video explanation from Johnny Betancourt
Start learning