Here are the essential concepts you must grasp in order to answer the question correctly.
Alkane Structure
Alkanes are saturated hydrocarbons consisting only of carbon (C) and hydrogen (H) atoms, connected by single bonds. The general formula for alkanes is CnH2n+2, where 'n' is the number of carbon atoms. Understanding the skeletal structure of alkanes is crucial, as it visually represents the arrangement of carbon atoms and their associated hydrogen atoms, which is essential for identifying the type of alkane being described.
Recommended video:
Secondary and Primary Carbons
In organic chemistry, carbon atoms can be classified based on the number of other carbon atoms they are bonded to. A secondary carbon is bonded to two other carbons, while a primary carbon is bonded to only one. This classification is important for constructing the skeletal structures requested in the question, as it determines the connectivity and branching of the carbon chain in the alkane.
Recommended video:
Secondary Protein Structure Example 2
Isopropyl Groups
An isopropyl group is a branched alkyl group derived from propane, consisting of three carbon atoms with the structure -C(CH3)2. When constructing skeletal structures, recognizing how to represent isopropyl groups is essential, especially when multiple groups are present, as in the case of the seven-carbon alkane with two isopropyl groups. This understanding aids in accurately depicting the molecular structure and ensuring correct connectivity.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution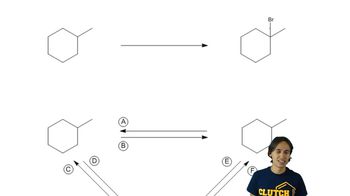



 6:42m
6:42m