Here are the essential concepts you must grasp in order to answer the question correctly.
Acidity and Acid Strength
Acidity refers to the ability of a compound to donate protons (H+) in solution. The strength of an acid is determined by its dissociation constant (Ka), which indicates how completely it ionizes in water. Stronger acids have higher Ka values, meaning they release protons more readily. In the context of benzoic acid and its derivatives, substituents can influence acidity by stabilizing or destabilizing the conjugate base formed after deprotonation.
Recommended video:
The 3 factors that determine the strength of inductive effects.
Inductive Effect
The inductive effect is the electron-withdrawing or electron-donating influence of substituents on a molecule through sigma bonds. Electron-withdrawing groups, such as nitro (NO2), can stabilize the negative charge on the conjugate base of an acid, thereby increasing its acidity. In ortho-substituted benzoic acids, the proximity of the nitro group enhances this effect, making these acids stronger than benzoic acid itself.
Recommended video:
Understanding the Inductive Effect.
Resonance Stabilization
Resonance stabilization occurs when a molecule can be represented by multiple valid Lewis structures, allowing for the delocalization of electrons. In the case of nitro-substituted benzoic acids, the resonance structures can help distribute the negative charge of the conjugate base more effectively. This delocalization further stabilizes the conjugate base, contributing to the increased acidity of ortho-nitrobenzoic acid compared to benzoic acid.
Recommended video:
The radical stability trend.

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution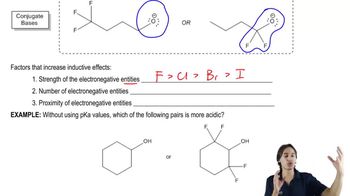



 5:39m
5:39m