Here are the essential concepts you must grasp in order to answer the question correctly.
Chair Conformation
The chair conformation is the most stable form of cyclohexane, allowing for minimized steric strain. In this conformation, carbon atoms are arranged in a staggered manner, which provides equatorial and axial positions for substituents. Understanding how substituents occupy these positions is crucial for analyzing the stability of disubstituted cyclohexanes.
Recommended video:
What is a chair conformation?
Equatorial vs. Axial Positions
In cyclohexane chair conformations, substituents can occupy equatorial positions, which extend outward from the ring, or axial positions, which are aligned parallel to the ring's axis. Equatorial positions generally lead to lower steric hindrance and greater stability, while axial positions can cause 1,3-diaxial interactions, increasing steric strain. Recognizing the implications of these positions is essential for determining the preferred conformer.
Recommended video:
Cis and Trans Isomerism
Cis and trans isomerism refers to the spatial arrangement of substituents on a cyclohexane ring. In cis isomers, substituents are on the same side of the ring, while in trans isomers, they are on opposite sides. This distinction affects the conformational analysis, as it influences whether substituents can both be equatorial or axial in the chair conformations, impacting the overall stability of the molecule.
Recommended video:
Is the following cyclohexane cis or trans?
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution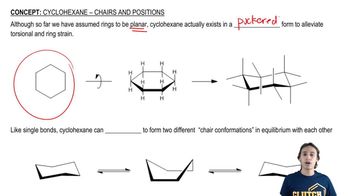



 1:05m
1:05m