Here are the essential concepts you must grasp in order to answer the question correctly.
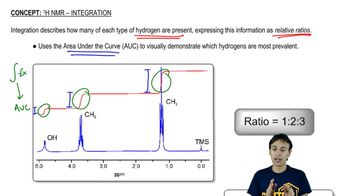
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It exploits the magnetic properties of certain nuclei, such as hydrogen (1H) and carbon (13C), to provide information about the number and environment of these atoms in a molecule. The resulting spectra reveal distinct signals that correspond to different chemical environments, aiding in the identification of molecular structures.
Recommended video:
1H NMR vs. 13C NMR
1H NMR focuses on the hydrogen atoms in a molecule, providing detailed information about the number of hydrogen environments and their connectivity. In contrast, 13C NMR targets carbon atoms, offering insights into the carbon skeleton and the types of functional groups present. The choice between the two depends on the specific structural features one aims to distinguish, as 1H NMR can provide more detailed information about hydrogen arrangements, while 13C NMR gives a broader view of the carbon framework.
Recommended video:
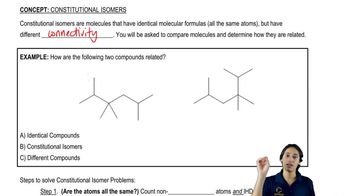
Isomer Distinction
Isomers are compounds with the same molecular formula but different structural arrangements. In the case of 1-butene, cis-2-butene, and 2-methylpropene, their distinct arrangements lead to different chemical environments for hydrogen and carbon atoms. Understanding how NMR can differentiate these isomers is crucial, as 1H NMR may reveal differences in hydrogen environments due to stereochemistry, while 13C NMR can highlight variations in carbon connectivity, making it essential to choose the appropriate technique for effective analysis.
Recommended video:
What is a constitutional isomer?
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 4:m
4:m