Here are the essential concepts you must grasp in order to answer the question correctly.
Conjugate Addition
Conjugate addition refers to the nucleophilic attack on an α,β-unsaturated carbonyl compound, where the nucleophile adds to the β-carbon rather than the α-carbon. This process is significant in organic synthesis as it allows for the formation of new carbon-carbon bonds. The stability of the resulting enolate or alkoxide intermediate is often enhanced by the presence of electron-withdrawing groups, which stabilize the negative charge through resonance.
Recommended video:
Resonance and Electron-Withdrawing Groups
Resonance is a concept in organic chemistry where a molecule can be represented by two or more valid Lewis structures, which contribute to the overall hybrid structure. Electron-withdrawing groups, such as cyano (–CN) and nitro (–NO2) groups, can stabilize negative charges through resonance, making the adjacent double bond more electrophilic. This stabilization increases the reactivity of the double bond towards nucleophiles, facilitating conjugate addition.
Recommended video:
Donating vs Withdrawing Groups
Mechanism of Nucleophilic Attack
The mechanism of nucleophilic attack involves the nucleophile donating a pair of electrons to the electrophilic carbon of the double bond, leading to the formation of a new bond. In the case of acrylonitrile and nitroethylene, the presence of the cyano and nitro groups enhances the electrophilicity of the double bond, allowing for a more favorable attack by the nucleophile. Understanding this mechanism is crucial for predicting the products of the reaction and the role of substituents in influencing reactivity.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution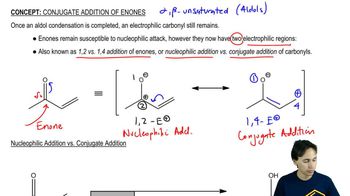



 9:23m
9:23m