Here are the essential concepts you must grasp in order to answer the question correctly.
Transesterification
Transesterification is a chemical reaction where an ester is converted into another ester through the exchange of the alkoxy group. This process typically involves the reaction of an alcohol with an ester in the presence of an acid catalyst, which enhances the reaction rate. Understanding this concept is crucial for analyzing the mechanism, as it outlines the fundamental transformation occurring in the reaction.
Recommended video:
Acid Catalysis
Acid catalysis involves the use of an acid to increase the rate of a chemical reaction by donating protons (H+) to reactants. In the context of transesterification, the acid catalyst protonates the carbonyl oxygen of the ester, making it more electrophilic and susceptible to nucleophilic attack by the alcohol. This concept is essential for understanding how the reaction proceeds and the role of the catalyst in stabilizing intermediates.
Recommended video:
Acid-Base Catalysis Concept 3
Resonance Stabilization
Resonance stabilization refers to the delocalization of electrons in a molecule, which can occur when multiple valid Lewis structures (resonance contributors) can be drawn. In the mechanism of acid-catalyzed transesterification, resonance stabilization plays a key role in stabilizing charged intermediates, such as the protonated ester. Recognizing these resonance contributors helps in predicting the stability and reactivity of intermediates throughout the reaction.
Recommended video:
The radical stability trend.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: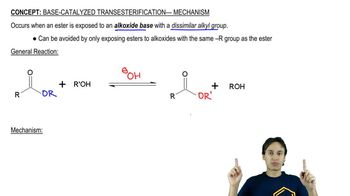



 2:49m
2:49m