Show how you would accomplish the following syntheses.
You may use whatever additional reagents you need.
(f) <IMAGE of reaction>

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

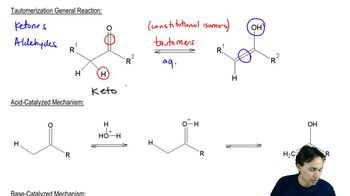

 3:19m
3:19mMaster Sodium Alkynide Alkylation with a bite sized video explanation from Johnny Betancourt
Start learning