Here are the essential concepts you must grasp in order to answer the question correctly.
D-Galactose Structure
D-galactose is a six-carbon aldose sugar with a specific stereochemistry. It contains an aldehyde functional group and multiple hydroxyl groups, which are crucial for its reactivity. Understanding its structure helps predict how it will interact with reagents, particularly in oxidation and substitution reactions.
Recommended video:
Monosaccharides - D and L Isomerism
Bromination Reaction
The reaction of sugars with bromine in the presence of water typically leads to the formation of bromohydrins. In this case, Br2 and H2O can oxidize the aldehyde group of d-galactose, resulting in the formation of a brominated sugar. This reaction is an example of electrophilic addition, where the bromine acts as an electrophile.
Recommended video:
Mechanism of Allylic Bromination.
Oxidation of Sugars
Oxidation of sugars involves the conversion of alcohols or aldehydes into carbonyl compounds or carboxylic acids. In the case of d-galactose reacting with Br2 and H2O, the aldehyde group can be oxidized to a carboxylic acid, leading to the formation of products like galactonic acid. This transformation is significant in carbohydrate chemistry and metabolic pathways.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution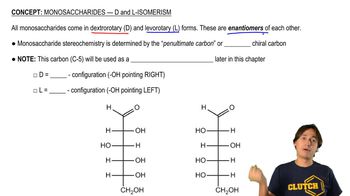



 4:15m
4:15m