Here are the essential concepts you must grasp in order to answer the question correctly.
Protic Solvents
Protic solvents are those that have a hydrogen atom attached to an electronegative atom, typically oxygen or nitrogen. This allows them to form hydrogen bonds and donate protons (H+ ions) in chemical reactions. Common examples include water and alcohols. Understanding whether a solvent is protic is crucial for predicting its behavior in reactions, especially in acid-base chemistry.
Recommended video:
The difference between protic vs. aprotic solvents.
Aprotic Solvents
Aprotic solvents lack hydrogen atoms that can participate in hydrogen bonding, meaning they do not donate protons. They can still dissolve ionic compounds and are often used in reactions where protic solvents would interfere. Examples include acetone and dimethyl sulfoxide (DMSO). Recognizing aprotic solvents is important for understanding their role in nucleophilic substitutions and other organic reactions.
Recommended video:
The difference between protic vs. aprotic solvents.
Solvent Properties and Reactivity
The properties of solvents significantly influence the reactivity and mechanism of organic reactions. Protic solvents can stabilize charged intermediates through solvation, while aprotic solvents can enhance nucleophilicity by not stabilizing anions as much. This distinction is vital for predicting reaction outcomes, especially in nucleophilic and electrophilic processes.
Recommended video:
Identification of polarity in solvents
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution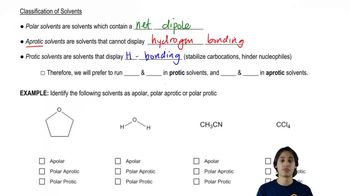



 2:26m
2:26m