Here are the essential concepts you must grasp in order to answer the question correctly.
Lone Pairs
Lone pairs are pairs of valence electrons that are not involved in bonding and are localized on a single atom. In organic chemistry, recognizing lone pairs is crucial for understanding molecular geometry, reactivity, and the overall electronic structure of molecules. They can influence the polarity and hydrogen bonding capabilities of a compound.
Recommended video:
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They provide a visual representation of the arrangement of electrons, helping to predict the shape and reactivity of the molecule. Drawing Lewis structures accurately is essential for identifying missing lone pairs and understanding molecular interactions.
Recommended video:
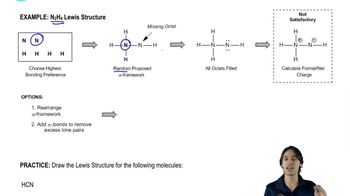
Drawing the Lewis Structure for N2H4.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to this theory, electron pairs, including lone pairs, will arrange themselves to minimize repulsion, which helps in determining the three-dimensional shape of the molecule. This understanding is vital when drawing structures and identifying lone pairs.
Recommended video:
Valence Electrons of Transition Metals

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:14m
2:14m