Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Formula
The molecular formula of a compound indicates the types and numbers of atoms present in a molecule. It is expressed as a combination of element symbols and numerical subscripts, such as CxHyOz, where x, y, and z represent the number of carbon, hydrogen, and oxygen atoms, respectively. Understanding how to derive the molecular formula is essential for identifying the composition of organic compounds.
Recommended video:
How to use IHD with molecular formula.
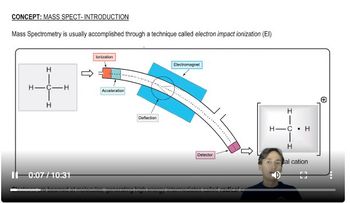
Mass-to-Charge Ratio (m/z)
The mass-to-charge ratio (m/z) is a crucial concept in mass spectrometry, representing the mass of an ion divided by its charge. In this context, an m/z value of 100 indicates that the molecular ion of the compound has a mass of 100 daltons. This information helps in deducing the molecular formula by providing a specific mass that the formula must correspond to.
Recommended video:
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In the case of a compound containing carbon (C), hydrogen (H), and oxygen (O), common functional groups include alcohols (–OH), aldehydes (–CHO), and ketones (C=O). Identifying the functional group can aid in predicting the compound's reactivity and properties, which is important for determining its molecular formula.
Recommended video:
Identifying Functional Groups
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:48m
1:48m