Here are the essential concepts you must grasp in order to answer the question correctly.
Ester Hydrolysis
Ester hydrolysis is a chemical reaction where an ester reacts with water to form an alcohol and a carboxylic acid. In the presence of a base, this reaction is often referred to as saponification. Understanding this process is crucial for determining which bonds are broken and which products are formed, especially when isotopes like O^18 are involved.
Recommended video:
Hydrolysis of Phosphate Esters Concept 2
Bond Selectivity in Hydrolysis
In ester hydrolysis, the selectivity of bond cleavage is significant. The acyl C—O bond is typically more reactive than the alkyl C—O bond under basic conditions. This concept is essential for understanding why the O^18 label appears in specific products, as it indicates which bond was broken during the reaction.
Recommended video:
Hydrolysis of Thioesters Concept 2
Isotope Labeling
Isotope labeling involves using isotopes, such as O^18, to trace the movement of atoms through a chemical reaction. In this context, it helps identify which products contain the labeled oxygen after hydrolysis. This technique is valuable for elucidating reaction mechanisms and understanding the pathways of chemical transformations.
Recommended video:
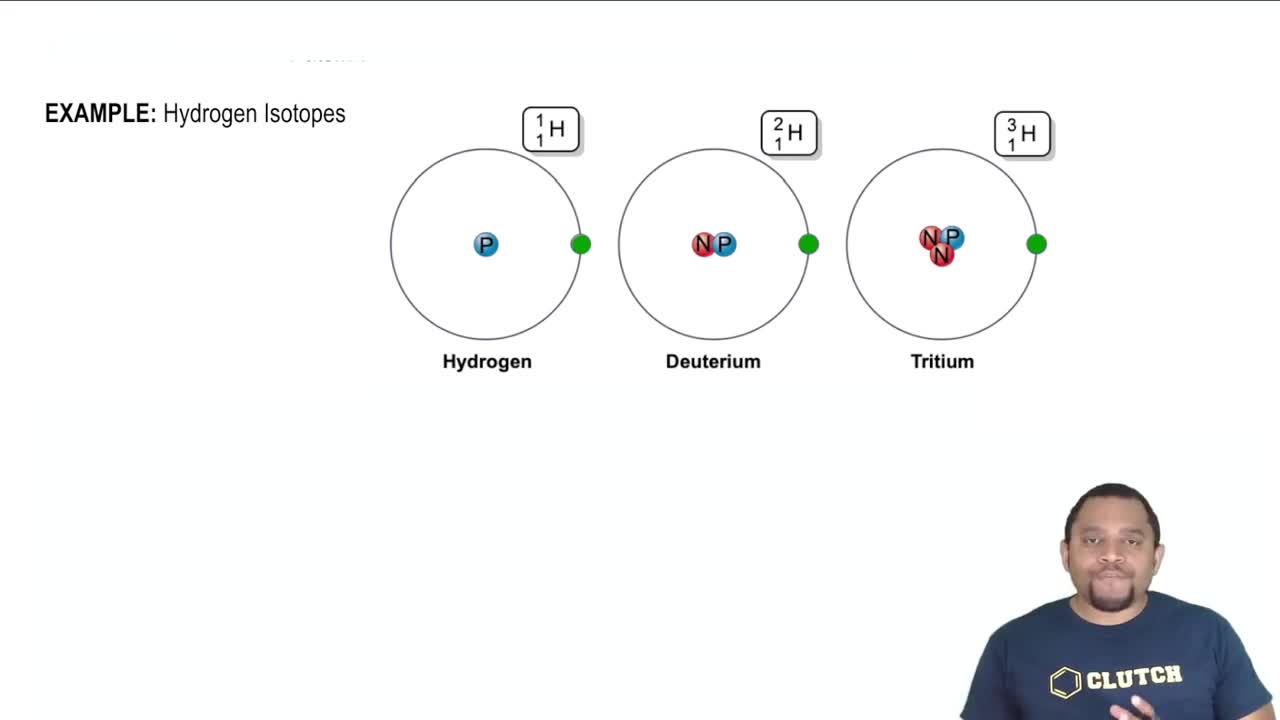
Understanding the hydrogen isotopes.

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution