Here are the essential concepts you must grasp in order to answer the question correctly.
NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It works by applying a magnetic field to nuclei of certain isotopes, such as hydrogen-1, causing them to resonate at specific frequencies. The resulting spectrum provides information about the number and environment of hydrogen atoms in a molecule, which is crucial for identifying functional groups and molecular structure.
Recommended video:
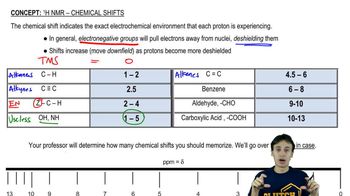
Chemical Shifts
Chemical shifts in NMR spectroscopy refer to the variation in resonance frequency of a nucleus due to its electronic environment. Measured in parts per million (ppm), these shifts help identify the type of hydrogen atoms present in a molecule. For example, protons attached to sp2 hybridized carbons, such as those in alkenes, typically resonate downfield (higher ppm values), which is evident in the spectrum of trans-hex-2-enoic acid around 7 ppm.
Recommended video:
Spin-Spin Coupling
Spin-spin coupling, or J-coupling, occurs when neighboring nuclei influence each other's magnetic environments, leading to splitting of NMR signals. The number of peaks in a signal can indicate the number of adjacent protons, following the n+1 rule, where n is the number of neighboring protons. Understanding this concept is essential for interpreting the complex splitting patterns observed in the vinyl protons of trans-hex-2-enoic acid, allowing for the estimation of coupling constants.
Recommended video:
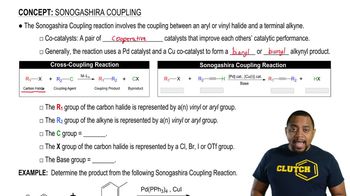
Sonogashira Coupling Reaction

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 8:02m
8:02m