Here are the essential concepts you must grasp in order to answer the question correctly.
1H NMR Spectroscopy
Proton Nuclear Magnetic Resonance (1H NMR) spectroscopy is a technique used to determine the structure of organic compounds by analyzing the magnetic environment of hydrogen atoms in a molecule. Each unique hydrogen environment produces a signal at a specific chemical shift (measured in ppm), allowing chemists to infer the number and type of protons present.
Recommended video:
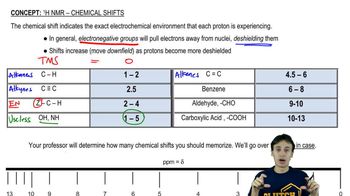
Chemical Shift
Chemical shift refers to the position of a signal in the NMR spectrum, which indicates the electronic environment surrounding the hydrogen atoms. Protons in different environments (e.g., near electronegative atoms or in aliphatic vs. aromatic regions) resonate at different frequencies, leading to distinct peaks in the spectrum that can be correlated to specific protons in the molecule.
Recommended video:
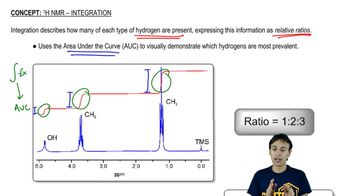
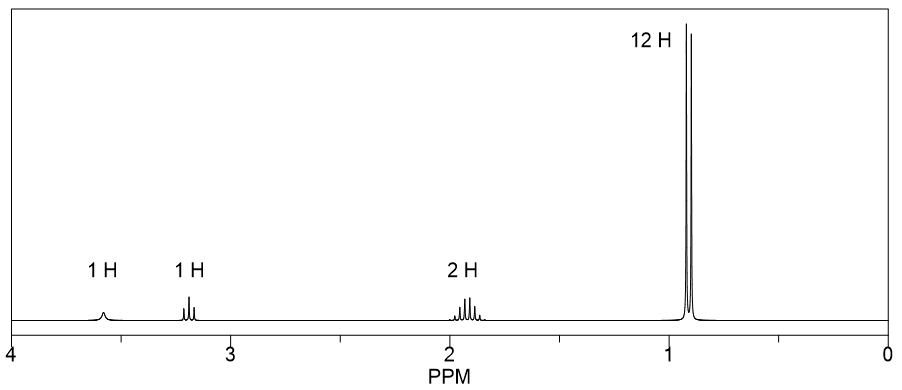
Integration and Multiplicity
Integration in NMR refers to the area under a peak, which corresponds to the number of protons contributing to that signal. Multiplicity indicates the splitting pattern of the peaks, which arises from neighboring protons (n+1 rule). Understanding these concepts helps in identifying how many protons are present and their connectivity in the molecular structure.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: