Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectric Point (pI)
The isoelectric point (pI) is the pH at which a molecule, such as an amino acid, carries no net electrical charge. For amino acids, this is crucial for understanding their behavior in electrophoresis, as they will migrate towards the electrode of opposite charge when the pH is below or above their pI. Knowing the pI helps predict the charge state of Trp, Cys, and His at pH 6.0.
Recommended video:
Definition of Isoelectric Point
Electrophoresis
Electrophoresis is a technique used to separate charged molecules, such as amino acids, based on their size and charge under an electric field. In this process, molecules migrate towards the electrode with the opposite charge, allowing for the separation of different species. The rate of migration depends on the charge-to-mass ratio of the molecules, which is influenced by the pH of the environment.
Amino Acid Side Chains
The side chains of amino acids, such as those in tryptophan (Trp), cysteine (Cys), and histidine (His), can be ionizable and affect the overall charge of the molecule at a given pH. At pH 6.0, the ionization states of these side chains determine their charge, influencing their migration during electrophoresis. Understanding the properties of these side chains is essential for predicting their behavior in an electric field.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution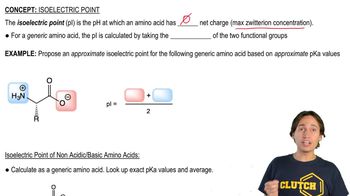


 3:19m
3:19m