Here are the essential concepts you must grasp in order to answer the question correctly.
Concerted Reactions
Concerted reactions are chemical processes where bond breaking and forming occur simultaneously in a single step, without intermediates. This mechanism is often characterized by a transition state where all reactants are involved at once. Understanding concerted reactions is crucial for predicting the outcome of reactions involving multiple bonds, such as those in 1,3-butadiene.
Recommended video:
Properties and Types of Pericyclic Reactions
Diels-Alder Reaction
The Diels-Alder reaction is a specific type of concerted reaction that involves a diene and a dienophile, leading to the formation of a cyclohexene derivative. In this case, 1,3-butadiene acts as the diene, while 2-cyclohexenone serves as the dienophile. This reaction is thermally allowed and can be influenced by light, making it essential to consider the conditions under which the reaction occurs.
Recommended video:
Diels-Alder Retrosynthesis
Ultraviolet Light in Organic Reactions
Ultraviolet (UV) light can provide the energy necessary to promote certain organic reactions, particularly those involving excited states or radical mechanisms. In the context of the question, UV light may facilitate the Diels-Alder reaction by promoting the diene or dienophile to a reactive state. Understanding the role of UV light helps in predicting whether a concerted reaction will proceed under specific conditions.
Recommended video:
Reaction with diazomethane and light or heat.
 Verified Solution
Verified Solution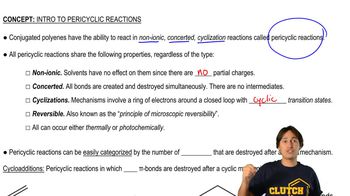



 9:36m
9:36m
