Here are the essential concepts you must grasp in order to answer the question correctly.
Cyclic Structures
Cyclic structures, such as cyclopentane, are closed-loop molecules where carbon atoms form a ring. This configuration can introduce strain and restrict the rotation around bonds due to the fixed positions of the atoms in the ring, leading to unique properties compared to linear alkanes like ethane.
Recommended video:
Monosaccharides - Forming Cyclic Hemiacetals
Bond Rotation and Steric Hindrance
In organic chemistry, bond rotation refers to the ability of atoms to rotate around a bond axis. In cyclopentane, steric hindrance occurs when atoms or groups are too close to each other, preventing free rotation and leading to a more rigid structure compared to alkanes, which can rotate freely around their single bonds.
Recommended video:
Single bonds, double bonds, and triple bonds.
Angle Strain
Angle strain arises when bond angles deviate from their ideal values, causing increased energy in a molecule. In cyclopentane, the ideal tetrahedral angle is 109.5 degrees, but the angles in the ring are slightly less, leading to angle strain that contributes to the restricted rotation and stability of the molecule.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: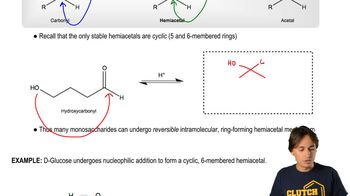



 3:29m
3:29m