Here are the essential concepts you must grasp in order to answer the question correctly.
Conrotatory vs. Disrotatory Ring Closure
In organic chemistry, the terms conrotatory and disrotatory refer to the specific types of stereochemical outcomes during the ring closure of conjugated systems under thermal conditions. Conrotatory closure involves the rotation of substituents in the same direction, while disrotatory closure involves rotation in opposite directions. The type of closure is determined by the number of π bonds and the stereochemistry of the reactants, which influences the product's configuration.
Recommended video:
Two Steps to Predicting Any Electrocyclic Products
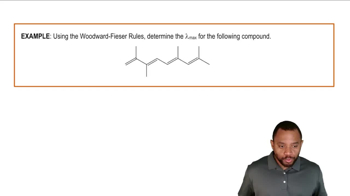
Woodward-Hoffmann Rules
The Woodward-Hoffmann rules provide a framework for predicting the stereochemical outcomes of pericyclic reactions, including cycloadditions and ring closures. These rules are based on the conservation of orbital symmetry and dictate whether a reaction will proceed via a conrotatory or disrotatory mechanism, depending on the number of π electrons involved. Understanding these rules is essential for determining the correct pathway and resulting stereochemistry of the product.
Recommended video:
Woodward-Fieser Rules Example 1
Cis and Trans Configurations
Cis and trans configurations refer to the spatial arrangement of substituents around a double bond or a ring structure. In a cis configuration, substituents are on the same side, while in a trans configuration, they are on opposite sides. The configuration significantly affects the physical and chemical properties of the compound, including its stability and reactivity, making it crucial to determine the correct configuration when analyzing the product of a reaction.
Recommended video:
Is the following cyclohexane cis or trans?
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 5:51m
5:51m