Here are the essential concepts you must grasp in order to answer the question correctly.
Lone Pairs
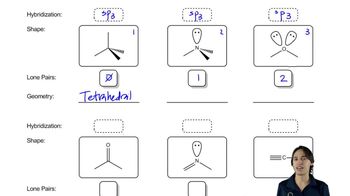
Lone pairs are pairs of valence electrons that are not involved in bonding and are localized on a single atom. In organic chemistry, recognizing lone pairs is crucial for understanding molecular geometry, reactivity, and the overall electronic structure of molecules. They can influence the shape of molecules and participate in hydrogen bonding, affecting physical properties.
Recommended video:
Line-Angle Drawings
Line-angle drawings, also known as skeletal structures, are a simplified way to represent organic molecules. In these drawings, vertices represent carbon atoms, and lines represent bonds between them. Hydrogen atoms attached to carbons are typically omitted for clarity, but it is essential to include lone pairs to fully depict the molecule's electronic structure and reactivity.
Recommended video:
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs around a central atom, influencing the molecule's shape and properties. Understanding molecular geometry is vital for predicting how molecules interact, their reactivity, and their physical properties.
Recommended video:
Molecular Geometry Explained.
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:14m
2:14m