Here are the essential concepts you must grasp in order to answer the question correctly.
Aldaric Acids
Aldaric acids are dicarboxylic acids derived from aldoses, formed when an aldose is oxidized at both the aldehyde and alcohol functional groups. The optical activity of these acids can vary based on the structure of the original sugar. In this case, the formation of an optically inactive aldaric acid indicates that the sugar has a symmetrical structure, while an optically active aldaric acid suggests asymmetry.
Recommended video:
Monosaccharides - Strong Oxidation (Aldaric Acid)
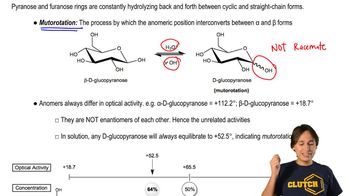
Optical Activity
Optical activity refers to the ability of a compound to rotate the plane of polarized light, which is a characteristic of chiral molecules. A sugar that is chiral will produce an optically active aldaric acid, while a sugar that is achiral will yield an optically inactive product. This property is crucial for distinguishing between glucose and galactose based on their oxidation products.
Recommended video:
Mutorotation and Optical Activity
Glucose vs. Galactose
Glucose and galactose are both hexose sugars but differ in the arrangement of hydroxyl groups around their carbon atoms, making them stereoisomers. Glucose is more symmetrical, leading to the formation of an optically inactive aldaric acid upon oxidation, while galactose, being less symmetrical, results in an optically active aldaric acid. This distinction is key to identifying which sugar corresponds to A and B in the question.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 3:10m
3:10m