Here are the essential concepts you must grasp in order to answer the question correctly.
Nucleophilic Substitution
Nucleophilic substitution is a fundamental reaction in organic chemistry where a nucleophile replaces a leaving group in a molecule. In the case of bromobenzene, the bromine atom acts as a leaving group, allowing nucleophiles like alkoxides or Grignard reagents to attack the aromatic ring, leading to the formation of new carbon-carbon or carbon-oxygen bonds.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Grignard Reagents
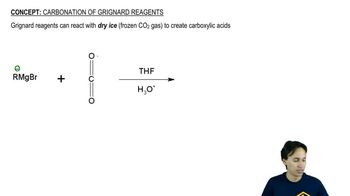
Grignard reagents are organomagnesium compounds that act as strong nucleophiles. They are formed by reacting an alkyl or aryl halide with magnesium in dry ether. In the synthesis of 1-phenylpropan-1-ol, a Grignard reagent derived from bromobenzene can be reacted with a suitable carbonyl compound, such as an aldehyde, to form the desired alcohol after hydrolysis.
Recommended video:
Carbonation of Grignard Reagents
Reduction Reactions
Reduction reactions involve the gain of electrons or the decrease in oxidation state of a molecule. In organic synthesis, alcohols can be produced by reducing carbonyl compounds. For synthesizing 1-phenylpropan-1-ol, a reduction step may be necessary if starting from a ketone or aldehyde, typically using reagents like lithium aluminum hydride or sodium borohydride.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 2:26m
2:26m