Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Shift
Chemical shift refers to the resonant frequency of a nucleus relative to a standard in a magnetic field, measured in parts per million (ppm). It provides insight into the electronic environment surrounding the nucleus, influenced by factors such as electronegativity and hybridization. In alkenes, hydrogen atoms typically resonate between 5 and 6 ppm due to their proximity to the double bond.
Recommended video:
Electronics and Substituent Effects
The electronic environment around a hydrogen atom can be altered by nearby substituents, which can either donate or withdraw electron density. This affects the chemical shift observed in NMR spectroscopy. For example, electron-withdrawing groups can deshield hydrogen atoms, causing them to resonate at lower ppm values, while electron-donating groups can have the opposite effect.
Recommended video:
Directing Effects in Substituted Pyrroles, Furans, and Thiophenes Concept 1
Steric Hindrance
Steric hindrance refers to the repulsion between atoms due to their physical size and spatial arrangement. In alkenes, bulky substituents can create steric strain, influencing the orientation and accessibility of hydrogen atoms. This can lead to variations in chemical shifts, as sterically hindered hydrogens may experience different electronic environments compared to more accessible ones.
Recommended video:
Understanding steric effects.
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution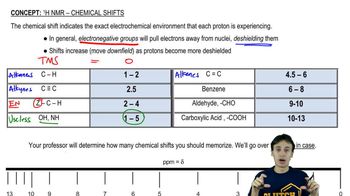



 11:44m
11:44m