Here are the essential concepts you must grasp in order to answer the question correctly.
Chair Conformation
The chair conformation is a three-dimensional representation of cyclohexane and its derivatives, which minimizes steric strain and torsional strain. In this conformation, the carbon atoms are arranged in a staggered manner, allowing for more stable interactions between substituents. Understanding chair conformations is crucial for visualizing the spatial arrangement of atoms in cyclic compounds.
Recommended video:
What is a chair conformation?
Cis-Trans Stereochemistry
Cis-trans stereochemistry refers to the orientation of substituents around a double bond or a ring structure. In cis isomers, substituents are on the same side, while in trans isomers, they are on opposite sides. This distinction is important in chair conformations, as it affects the relative positions of substituents and their steric interactions, influencing the stability of the molecule.
Recommended video:
Is the following cyclohexane cis or trans?
Stereoisomerism
Stereoisomerism is a form of isomerism where molecules have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. This includes both geometric isomers (like cis and trans) and optical isomers. Recognizing stereoisomers is essential for understanding the reactivity and properties of organic compounds, particularly in cyclic structures.
Recommended video:
Determining when molecules are stereoisomers.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: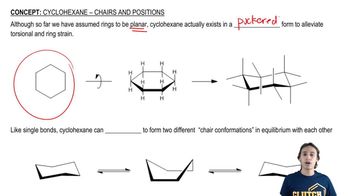



 1:18m
1:18m