Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It exploits the magnetic properties of certain nuclei, such as carbon-13 (¹³C), to provide information about the environment surrounding these nuclei. In ¹³C NMR, the number of signals corresponds to the number of unique carbon environments in a molecule, allowing chemists to deduce structural features.
Recommended video:
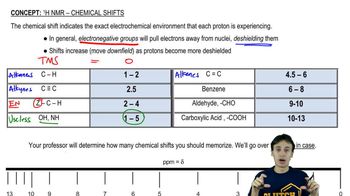
Chemical Shift
Chemical shift refers to the variation in the resonance frequency of a nucleus due to its electronic environment. In ¹³C NMR, chemical shifts are measured in parts per million (ppm) and provide insight into the types of carbon atoms present, such as whether they are part of an alkane, alkene, or aromatic system. Understanding chemical shifts is crucial for interpreting NMR spectra and assigning signals to specific carbon atoms in a molecule.
Recommended video:
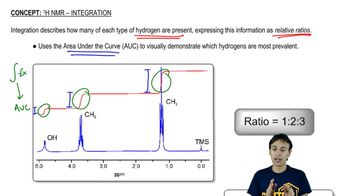
Integration and Multiplicity
Integration in NMR refers to the area under a signal, which correlates with the number of nuclei contributing to that signal. In ¹³C NMR, while integration is less commonly used than in ¹H NMR, it can still provide insights into the relative number of equivalent carbons. Multiplicity, on the other hand, indicates the number of neighboring hydrogen atoms affecting the carbon signal, although in ¹³C NMR, signals are typically singlets due to the low natural abundance of ¹³C and the lack of direct coupling with protons.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 4:m
4:m