Here are the essential concepts you must grasp in order to answer the question correctly.
Sonogashira Coupling
The Sonogashira coupling is a cross-coupling reaction that involves the coupling of an aryl or vinyl halide with an alkyne in the presence of a palladium catalyst and a base. This reaction is widely used in organic synthesis to form carbon-carbon bonds, particularly in the construction of complex molecules and materials. Understanding the mechanism and conditions of this reaction is crucial for predicting the products formed.
Recommended video:
Sonogashira Coupling Reaction
Palladium Catalysis
Palladium catalysis is a key component in many cross-coupling reactions, including the Sonogashira reaction. Palladium serves as a catalyst that facilitates the formation of the carbon-carbon bond by activating the halide and the alkyne. The choice of palladium source and the reaction conditions can significantly influence the efficiency and selectivity of the reaction, making it essential to understand its role in product formation.
Recommended video:
Nucleophilic Catalysis Concept 1
Reaction Conditions
The reaction conditions, including temperature, solvent, and the presence of a base, play a critical role in the Sonogashira coupling. Typically, a base is required to deprotonate the alkyne, facilitating the formation of the nucleophilic species that reacts with the palladium-activated halide. Adjusting these conditions can affect the yield and purity of the desired product, making it important to consider them when predicting the outcome of the reaction.
Recommended video:
EAS Reactions of Pyridine Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution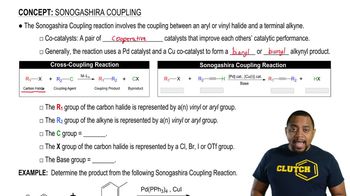


 2:54m
2:54m