Here are the essential concepts you must grasp in order to answer the question correctly.
Mass Spectrometry Fragmentation
Mass spectrometry fragmentation involves the breaking of chemical bonds in a molecule to produce smaller ions, which can be detected and analyzed. The pattern of these fragments provides insight into the structure of the original compound. Each fragment corresponds to a specific mass-to-charge ratio (m/z), allowing chemists to deduce the molecular structure based on the observed peaks in the mass spectrum.
Recommended video:
How to Read a Mass Spectrum
Ethers and Their Structure
Ethers are organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups. Their general structure is R-O-R', where R and R' can be the same or different. The unique structural features of ethers influence their fragmentation patterns in mass spectrometry, leading to distinctive peaks that can help differentiate between similar compounds.
Recommended video:
Interpreting Mass Spectra Peaks
Interpreting mass spectra peaks involves analyzing the m/z values to identify the corresponding fragments and their origins. Each peak represents a specific ion formed during fragmentation, and the intensity of the peaks indicates the relative abundance of those ions. By comparing the peaks at m/z 116, 101, 87, and 73, one can deduce which compound produces which peak and propose possible fragmentation pathways.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution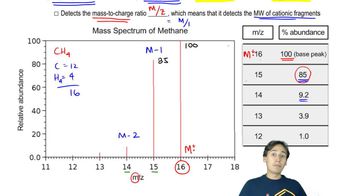



 4:28m
4:28m