Here are the essential concepts you must grasp in order to answer the question correctly.
Kiliani–Fischer Synthesis
The Kiliani–Fischer synthesis is a chemical reaction used to elongate monosaccharides by adding a carbon atom to the chain. This process involves the formation of an aldehyde from a sugar, followed by the addition of cyanide to create a new carbon-carbon bond. The reaction ultimately leads to the formation of two new aldoses, which can be further hydrolyzed to yield different monosaccharides.
Recommended video:
Monosaccharides - Kiliani-Fischer
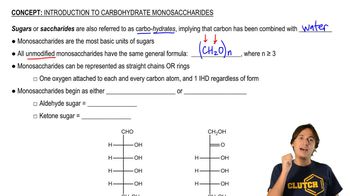
Monosaccharides
Monosaccharides are the simplest form of carbohydrates, consisting of single sugar units that cannot be hydrolyzed into simpler sugars. Common examples include glucose, fructose, and xylose. They serve as the building blocks for more complex carbohydrates and play crucial roles in energy metabolism and cellular functions.
Recommended video:
Stereochemistry of Sugars
Stereochemistry refers to the spatial arrangement of atoms in molecules and is particularly important in sugars due to the presence of multiple chiral centers. The configuration of these centers determines the specific properties and reactivity of the sugars. In the context of the Kiliani–Fischer synthesis, understanding the stereochemistry of the starting monosaccharide, such as d-xylose, is essential for predicting the structure of the resulting products.
Recommended video:
Polymer Stereochemistry Concept 1