Here are the essential concepts you must grasp in order to answer the question correctly.
Amide Reactivity
Amides are generally less reactive than other carbonyl compounds due to the resonance stabilization provided by the nitrogen atom. The lone pair of electrons on the nitrogen can delocalize into the carbonyl group, reducing the electrophilicity of the carbonyl carbon. Understanding the factors that influence amide reactivity, such as steric hindrance and electronic effects, is crucial for predicting their behavior in reactions like acid-catalyzed hydrolysis.
Recommended video:
Electronic Effects
The electronic environment around the carbonyl group significantly affects the reactivity of amides. Electron-withdrawing groups, such as nitro groups, increase the electrophilicity of the carbonyl carbon, making the amide more reactive. Conversely, electron-donating groups can decrease reactivity by stabilizing the carbonyl through resonance. Analyzing the substituents on the amides in the question helps determine their relative reactivity in hydrolysis.
Recommended video:
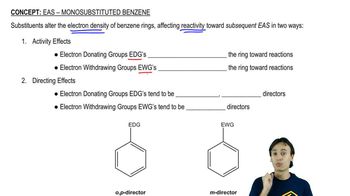
Activity and Directing Effects
Steric Hindrance
Steric hindrance refers to the crowding around a reactive site that can impede the approach of reactants. In the context of amides, bulky substituents near the carbonyl group can hinder the access of water molecules during acid-catalyzed hydrolysis, thus reducing reactivity. Evaluating the steric effects of the substituents in the given amides is essential for ranking their reactivity accurately.
Recommended video:
Understanding steric effects.

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution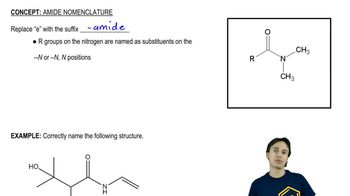


 5:39m
5:39m