Here are the essential concepts you must grasp in order to answer the question correctly.
Stereoisomerism
Stereoisomerism refers to the phenomenon where compounds have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of their atoms. This can lead to different physical and chemical properties. In organic chemistry, stereoisomers can be classified into enantiomers and diastereomers, which are crucial for understanding the diversity of compounds formed in reactions.
Recommended video:
Determining when molecules are stereoisomers.
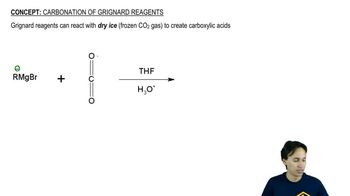
Grignard Reagents
Grignard reagents are organomagnesium compounds that are highly reactive and used to form carbon-carbon bonds. In this case, ethylmagnesium bromide reacts with 2-pentanone, leading to the formation of a tertiary alcohol after the addition of dilute acid. Understanding the reactivity and mechanism of Grignard reagents is essential for predicting the products of such reactions.
Recommended video:
Carbonation of Grignard Reagents
Chiral Centers
A chiral center, typically a carbon atom, is bonded to four different substituents, leading to non-superimposable mirror images known as enantiomers. The formation of a chiral center during the reaction of 2-pentanone with ethylmagnesium bromide results in the potential for multiple stereoisomers. Identifying chiral centers is key to determining the number of stereoisomers produced in a reaction.
Recommended video:
Understanding Other Chiral Atoms
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 3:57m
3:57m