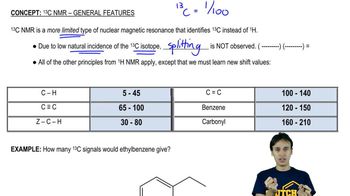
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR)
Nuclear Magnetic Resonance (NMR) is a spectroscopic technique used to observe the magnetic properties of atomic nuclei. In NMR, nuclei in a magnetic field absorb and re-emit electromagnetic radiation, allowing for the determination of molecular structure. The frequency of the NMR spectrometer, measured in megahertz (MHz), indicates the strength of the magnetic field and influences the energy required to flip the nuclear spin states.
Recommended video:
Energy Calculation in NMR
The energy required to flip a nucleus in NMR can be calculated using the formula E = hν, where E is energy, h is Planck's constant, and ν is the frequency of the NMR spectrometer. This relationship shows that higher frequencies correspond to higher energy transitions. For a spectrometer operating at 300 MHz, this calculation will yield the energy in joules, which can then be converted to calories for the final answer.
Recommended video:
Conversion of Energy Units
In scientific calculations, it is often necessary to convert energy units from joules to calories. The conversion factor is 1 calorie = 4.184 joules. Understanding this conversion is essential for expressing the energy required for the NMR process in the desired units, ensuring clarity and accuracy in the final result.
Recommended video:
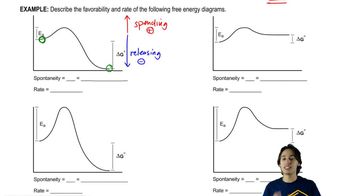
Favorability and rate of Free Energy Diagrams