Here are the essential concepts you must grasp in order to answer the question correctly.
Proton NMR Spectroscopy
Proton Nuclear Magnetic Resonance (¹H NMR) spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It works by measuring the magnetic environment of hydrogen atoms in a molecule, providing information about the number of hydrogen atoms, their chemical environment, and the connectivity between atoms. The resulting spectrum displays peaks that correspond to different hydrogen environments, allowing chemists to infer structural details.
Recommended video:
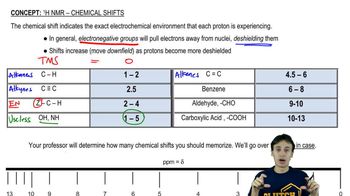
Chemical Shifts
Chemical shifts in ¹H NMR refer to the position of the peaks in the spectrum, which are influenced by the electronic environment surrounding the hydrogen atoms. Different functional groups and molecular structures cause shifts in the resonance frequency of hydrogen atoms, typically measured in parts per million (ppm). Understanding chemical shifts is crucial for predicting how the product's spectrum will differ from that of the reactant, as they indicate the presence of different functional groups or changes in hybridization.
Recommended video:
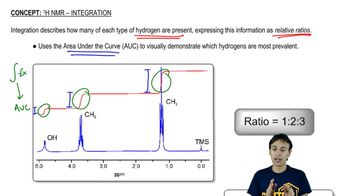
Integration and Multiplicity
Integration and multiplicity are key features of ¹H NMR spectra that provide insights into the number of hydrogen atoms and their interactions. Integration refers to the area under the peaks, which correlates with the number of protons contributing to that signal. Multiplicity, determined by the splitting of peaks, reveals how many neighboring hydrogen atoms are present, following the n+1 rule. Together, these concepts help in deducing the connectivity and arrangement of atoms in the product structure.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: