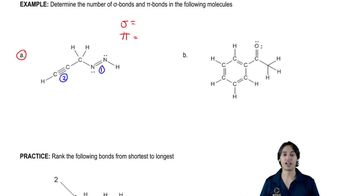
Textbook Question
(b) Show the electronic configuration of the ground state of hexa-1,3,5-triene.
514
views
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 3:44m
3:44mMaster The 7 Rules of Drawing Molecular Orbitals with a bite sized video explanation from Johnny Betancourt
Start learning