Here are the essential concepts you must grasp in order to answer the question correctly.
Bromine Water as an Oxidizing Agent
Bromine water is a solution of bromine in water that acts as a mild oxidizing agent. It can oxidize alcohols and aldehydes to their corresponding carbonyl compounds, and in the case of sugars, it can oxidize certain functional groups, leading to the formation of carboxylic acids. Understanding its reactivity is crucial for predicting the products of the oxidation of carbohydrates.
Recommended video:
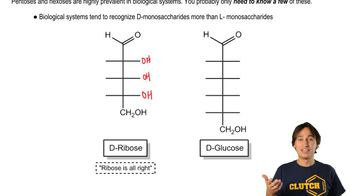
Monosaccharide Structure and Reactivity
Monosaccharides like D-mannose, D-galactose, and D-fructose have distinct structures that influence their reactivity. D-mannose and D-galactose are aldoses, containing aldehyde groups, while D-fructose is a ketose, containing a ketone group. The presence of these functional groups determines how each sugar will react with bromine water, leading to different oxidation products.
Recommended video:
Monosaccharides - Common Structures
Product Identification in Oxidation Reactions
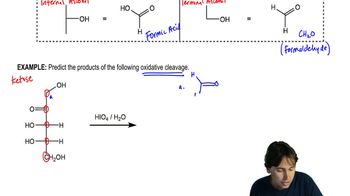
Identifying the products of oxidation reactions involves understanding the transformation of functional groups. For aldoses, oxidation typically results in the formation of carboxylic acids, while ketoses may yield different products. Recognizing these transformations is essential for accurately drawing and naming the products of the bromine water oxidation of the specified sugars.
Recommended video:
Predict the products of the following oxidative cleavage
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 4:15m
4:15m