Here are the essential concepts you must grasp in order to answer the question correctly.
Ethanol Dehydration
Ethanol dehydration is a chemical reaction where ethanol (an alcohol) loses a water molecule to form an ether or alkene. In the presence of an acid catalyst, such as sulfuric acid, the hydroxyl group (-OH) is protonated, making it a better leaving group. This reaction typically requires heat to proceed, facilitating the elimination of water and the formation of a double bond or ether linkage.
Recommended video:
General Reaction of Dehydration with POCl3
Acid-Catalyzed Ether Formation
Acid-catalyzed ether formation involves the reaction of two alcohol molecules in the presence of an acid catalyst, leading to the formation of an ether. The acid protonates the alcohol, enhancing its reactivity. The resulting alkyl oxonium ion can then undergo nucleophilic attack by another alcohol molecule, resulting in the formation of an ether and the release of water.
Recommended video:
Mechanism of Nucleophilic Substitution
The mechanism of nucleophilic substitution is a fundamental concept in organic chemistry where a nucleophile attacks an electrophile, resulting in the replacement of a leaving group. In the context of ether formation, the alcohol acts as a nucleophile, attacking the electrophilic carbon of the alkyl oxonium ion. This process can follow either an SN1 or SN2 pathway, depending on the structure of the reactants and the conditions of the reaction.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution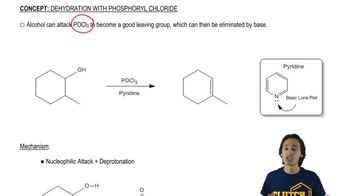


 4:33m
4:33m