Here are the essential concepts you must grasp in order to answer the question correctly.
Suzuki Reaction
The Suzuki reaction is a widely used cross-coupling reaction in organic chemistry that allows for the formation of biaryl compounds. It involves the coupling of an aryl halide with an organoboron compound in the presence of a palladium catalyst and a base. This reaction is particularly valued for its ability to create carbon-carbon bonds with high selectivity and efficiency, making it essential for synthesizing complex organic molecules.
Recommended video:
Aryl Halides
Aryl halides are organic compounds that contain a halogen atom (such as chlorine, bromine, or iodine) bonded to an aromatic ring. They serve as key starting materials in various organic reactions, including the Suzuki reaction. The reactivity of aryl halides is influenced by the nature of the halogen and the electronic properties of the aromatic system, which can affect the efficiency of the coupling process.
Recommended video:
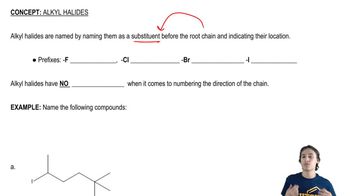
How to name alkyl halides
Organoboron Compounds
Organoboron compounds, such as boronic acids and boronate esters, are crucial reagents in the Suzuki reaction. They contain a boron atom bonded to carbon and are typically more stable and less toxic than other organometallic reagents. Their ability to undergo transmetallation with palladium facilitates the formation of carbon-carbon bonds, making them essential for synthesizing biaryl compounds in a variety of chemical contexts.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 4:02m
4:02m