Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Shift in NMR Spectroscopy
Chemical shift refers to the resonance frequency of a nucleus relative to a standard in a magnetic field, typically measured in parts per million (ppm). In proton NMR, the chemical shift is influenced by the electronic environment surrounding the hydrogen atoms, with electronegative atoms or groups causing downfield shifts (higher ppm) due to deshielding effects.
Recommended video:
Deshielding and Shielding Effects
Shielding occurs when surrounding electrons reduce the magnetic field experienced by a nucleus, resulting in a lower chemical shift. Conversely, deshielding happens when electronegative atoms withdraw electron density from the hydrogen, increasing the chemical shift. Understanding these effects is crucial for predicting which protons will resonate at higher frequencies in an NMR spectrum.
Recommended video:
Influence of Substituents on Chemical Shift
Different substituents attached to a carbon chain can significantly affect the chemical shifts of protons. For example, halogens like Cl and Br, and functional groups like -OH or carbonyls, can cause varying degrees of deshielding. Analyzing the molecular structure and identifying these substituents helps in determining which protons will exhibit greater chemical shifts in the NMR spectrum.
Recommended video:

 Verified Solution
Verified Solution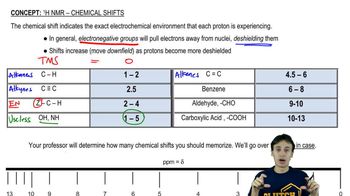



 11:44m
11:44m