Here are the essential concepts you must grasp in order to answer the question correctly.
Infrared Spectroscopy (IR)
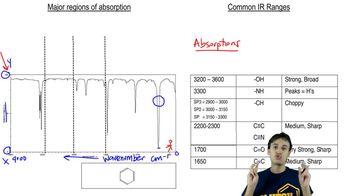
Infrared spectroscopy is a technique used to identify molecular structures based on the absorption of infrared light by chemical bonds. Different functional groups absorb IR radiation at characteristic wavenumbers, allowing chemists to deduce the presence of specific bonds, such as C-O. The position of these absorption bands is influenced by factors like bond strength and molecular environment.
Recommended video:
General Features of IR Spect
C-O Bond Characteristics
The C-O bond can exist in various functional groups, such as alcohols, ethers, and carbonyls, each exhibiting different absorption wavenumbers in IR spectroscopy. Generally, stronger bonds and those in more polar environments absorb at higher wavenumbers. For example, carbonyl (C=O) groups typically show strong absorption around 1700 cm⁻¹, while alcohols (C-O) absorb at lower wavenumbers, around 1000-1300 cm⁻¹.
Recommended video:
Single bonds, double bonds, and triple bonds.
Factors Affecting Wavenumber
The wavenumber of an absorption band in IR spectroscopy is influenced by several factors, including bond strength, hybridization, and molecular interactions. Stronger bonds, such as double bonds, absorb at higher wavenumbers compared to single bonds. Additionally, resonance and steric effects can shift the absorption frequency, making it essential to consider the molecular structure when ranking compounds by their C-O absorption bands.
Recommended video:
Using factors affecting acidity to rank acids