Here are the essential concepts you must grasp in order to answer the question correctly.
Anomeric Carbon
The anomeric carbon is the carbon atom in a sugar molecule that is derived from the carbonyl carbon of the open-chain form. In cyclic forms of sugars, it is the carbon that determines the alpha or beta configuration, depending on the orientation of the hydroxyl group attached to it. Understanding this concept is crucial for drawing the correct structures of the cyclic forms of d-glucose.
Recommended video:
Carbonation of Grignard Reagents
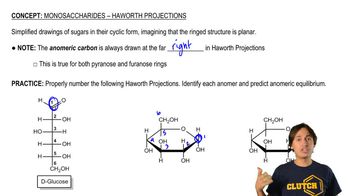
Haworth Projection
The Haworth projection is a common way to represent the cyclic forms of sugars, showing the ring structure in a two-dimensional format. It illustrates the orientation of substituents around the ring, which is essential for visualizing the stereochemistry of the sugar. This representation helps in understanding the equilibrium between the open-chain and cyclic forms of d-glucose.
Recommended video:
Monosaccharides - Haworth Projections
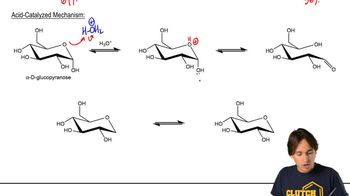
Mutarotation
Mutarotation is the process by which the specific rotation of a sugar solution changes over time as it equilibrates between its anomeric forms. For d-glucose, this involves the interconversion between the alpha and beta anomers in solution. Recognizing this phenomenon is important for understanding the dynamic nature of sugar structures in aqueous environments.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution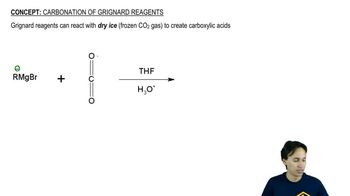

 12:58m
12:58m