Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom or ion. For metals, this energy is typically lower, allowing them to lose electrons easily and form positive ions. Lithium, being an alkali metal, has a relatively low ionization energy, which facilitates its ionization to form a cation.
Recommended video:
Cation Formation
Cation formation occurs when an atom loses one or more electrons, resulting in a positively charged ion. In the case of lithium, it tends to lose one electron to achieve a stable electron configuration similar to that of noble gases. This process is crucial for understanding the behavior of metals in chemical reactions.
Recommended video:
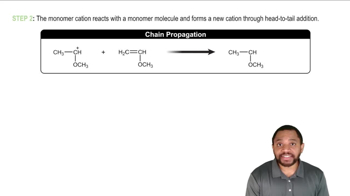
Cationic Polymerization Concept 3
Charge of Alkali Metals
Alkali metals, including lithium, typically form cations with a +1 charge when they ionize. This is due to their single valence electron, which they readily lose to achieve a stable electronic configuration. Understanding this characteristic is essential for predicting the behavior of lithium in chemical reactions and its interactions with other elements.
Recommended video:
Metal Ion Catalysis: Water Activation Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:14m
2:14m