Here are the essential concepts you must grasp in order to answer the question correctly.
Acidic Hydrolysis
Acidic hydrolysis of an ester involves the addition of water in the presence of an acid catalyst, typically a strong acid like hydrochloric acid. The acid donates protons (H+) to the ester, enhancing the electrophilicity of the carbonyl carbon, which facilitates the nucleophilic attack by water. This process leads to the formation of a carboxylic acid and an alcohol.
Recommended video:
Hydrolysis of Nucleosides Concept 1
Basic Hydrolysis
Basic hydrolysis, often referred to as saponification, occurs when an ester reacts with a strong base, such as sodium hydroxide. In this reaction, the hydroxide ion (OH-) acts as a nucleophile, attacking the carbonyl carbon of the ester. This process is termed 'base-promoted' because the base is not merely a catalyst; it is consumed in the reaction, resulting in the formation of a carboxylate ion and an alcohol.
Recommended video:
Understanding the difference between basicity and nucleophilicity.
Catalysis vs. Promotion
The distinction between 'catalyzed' and 'promoted' reactions lies in the role of the reactants. In acid-catalyzed reactions, the acid is not consumed and can be reused, acting solely to speed up the reaction. In contrast, in base-promoted reactions, the base is consumed in the reaction, leading to the formation of products, which is why we refer to it as 'promoted' rather than 'catalyzed.'
Recommended video:
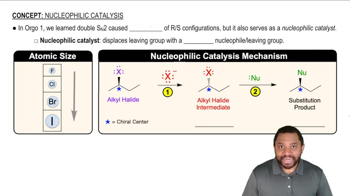
Nucleophilic Catalysis Concept 1