Here are the essential concepts you must grasp in order to answer the question correctly.
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (EAS) is a fundamental reaction in organic chemistry where an electrophile replaces a hydrogen atom on an aromatic ring. This mechanism is crucial for converting aniline to halogenated derivatives like fluorobenzene and chlorobenzene. The aromatic ring's electron density attracts electrophiles, allowing for the substitution to occur while maintaining the aromaticity of the compound.
Recommended video:
Reactivity of Aniline
Aniline, an aromatic amine, is more reactive than benzene due to the electron-donating effect of the amino group (-NH2). This increased reactivity facilitates the electrophilic substitution reactions, making it easier to introduce halogens onto the aromatic ring. Understanding how the amino group influences the reactivity and orientation of substitution is essential for predicting the products formed during the conversion.
Recommended video:
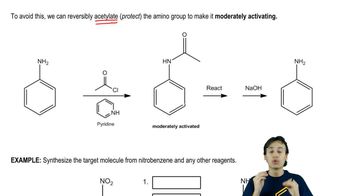
Protection of Aniline Derivatives
Halogenation Reagents
The conversion of aniline to fluorobenzene and chlorobenzene requires specific halogenation reagents. For chlorination, reagents like chlorine gas (Cl2) in the presence of a catalyst (e.g., FeCl3) are commonly used. For fluorination, more reactive reagents such as fluorine gas (F2) or electrophilic fluorinating agents are necessary due to the high reactivity of fluorine. Knowing the appropriate reagents and conditions is vital for successful halogenation.
Recommended video: