Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Mechanism
A reaction mechanism is a step-by-step description of how a chemical reaction occurs at the molecular level. It outlines the sequence of elementary steps, including bond breaking and formation, and the intermediates formed during the process. Understanding the mechanism is crucial for predicting the products and the conditions under which the reaction occurs.
Recommended video:
Nucleophiles and Electrophiles
Nucleophiles are species that donate an electron pair to form a chemical bond, while electrophiles are electron-deficient species that accept an electron pair. In organic reactions, identifying the nucleophile and electrophile is essential for understanding how they interact and lead to product formation. This concept is fundamental in predicting the outcome of many organic reactions.
Recommended video:
Nucleophile or Electrophile
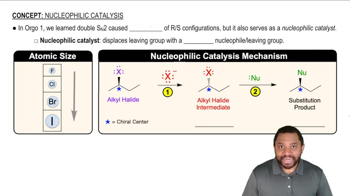
Catalysis
Catalysis refers to the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst, which is not consumed in the reaction. Catalysts can lower the activation energy required for a reaction, making it proceed more efficiently. Understanding the role of catalysts is important for analyzing reaction mechanisms and optimizing reaction conditions.
Recommended video:
Nucleophilic Catalysis Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:49m
2:49m