Here are the essential concepts you must grasp in order to answer the question correctly.
Spin States
Spin states refer to the intrinsic angular momentum of particles, such as electrons, which can exist in different orientations. For a hydrogen atom, the spin can be either 'up' or 'down', leading to various combinations when considering neighboring atoms. The total number of spin states is crucial for understanding the resulting magnetic interactions in NMR spectroscopy.
Recommended video:
Ground vs. Excited States
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a technique used to observe the magnetic properties of atomic nuclei. In the context of hydrogen atoms, the presence of neighboring hydrogen atoms influences the splitting of NMR signals, known as spin-spin coupling. The number of neighboring hydrogen atoms determines the multiplicity of the signal, which is observed as peaks in the spectrum.
Recommended video:
Multiplicity and Coupling
Multiplicity refers to the number of peaks in an NMR signal, which is determined by the number of neighboring hydrogen atoms plus one (n+1 rule). When a hydrogen atom has four neighboring hydrogens, it results in a quintet, indicating five distinct peaks. This phenomenon occurs due to the coupling interactions between the spins of the hydrogen atoms, leading to a specific pattern in the NMR spectrum.
Recommended video:
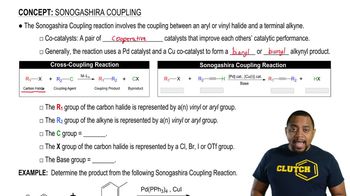
Sonogashira Coupling Reaction
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 12:21m
12:21m